SillaJen and collaboration partner Transgene today separately acknowledged the failure of their lead pipeline candidate Pexa-Vec (pexastimogene devacirepvec) in a Phase III trial assessing a combination of the oncolytic virus and the Bayer/Amgen cancer drug Nexavar® (sorafenib) in liver cancer—a clinical setback that sent the shares of Pexa-Vec’s developers tumbling.
South-Korea-based SillaJen said in a regulatory filing that it was advised to halt patient enrollment in the Phase III PHOCUS trial (NCT02562755) by the study’s Independent Data Monitoring Committee (IDMC) following completion of a planned interim futility analysis.
According to SillaJen and Transgene—which owns development and commercialization rights to Pexa-Vec in Europe—the analysis showed that PHOCUS was unlikely to meet its primary objective by the time of the final analysis, namely overall survival from the date of randomization to the date of death due to any cause up to study completion, a timeframe of approximately 53 months. The companies have not released data from PHOCUS.
No safety concerns have been reported by SillaJen. Transgene said it was informed by SillaJen of the IDMC recommendation, and will update investors in an upcoming conference call.
PHOCUS is a multi-center, randomized, open-label, study designed to compare Pexa Vec followed by Nexavar versus Nexavar alone in up to 600 patients with advanced hepatocellular carcinoma (HCC) without prior systemic therapy.
In September 2018, SillaJen joined Lee’s Pharmaceutical Holdings—which holds China and Hong Kong rights to Pexa-Vec—in announcing the first patient enrolled in PHOCUS. The trial was launched following positive Phase II data showing statistically significant improved overall survival for advanced liver cancer patients receiving a high dose of Pexa-Vec (1e9 plaque forming units of pfu) compared to the group receiving the low dose (1e8 pfu)—14.1 months compared with 6.7 months.
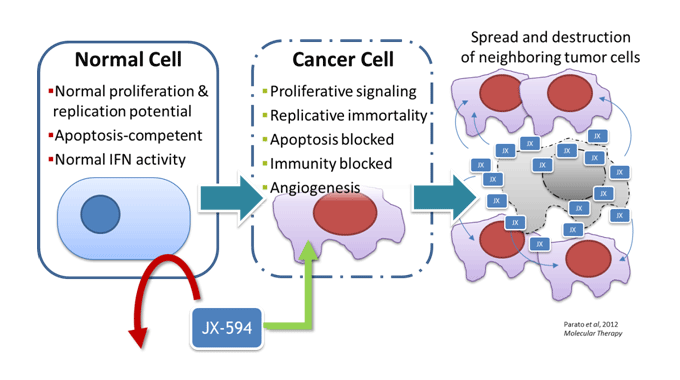
Pexa-Vec, also known as JX-594, is a Wyeth strain oncolytic vaccinia virus armed with a granulocyte-macrophage colony stimulating factor (GM-CSF) gene designed to promote an anti-tumor immune response. According to SillaJen, Pexa-Vec is designed to fight cancer by:
- Infecting and selectively replicating in cancer cells and causing lysis
- Reducing the blood supply to tumors through infection of tumor associate vasculature
- Activating the body’s own immune system to recognize and kill tumor cells.
According to SillaJen, Pexa-Vec replication and spread are dependent on activation of the EGFR/Raf/Ras signaling pathway and high cellular levels of thymidine kinase (TK) found in proliferating cancer cells.
“Up-and-Coming”
Pexa-Vec appeared on GEN’s A-List of 25 Up and Coming Gene Therapies of 2019, published May 20, and made last year’s A-List as well. The list includes gene-therapy candidates that have reached Phase III and/or registrational trials as of the first quarter of 2019, according to the Alliance for Regenerative Medicine (ARM), as well as ClinicalTrials.gov and/or company announcements.
In addition to PHOCUS, Pexa-Vec is also in a Phase I/II trial (NCT03071094) assessing a combination of the oncolytic virus with Bristol-Myers Squibb’s marketed cancer immunotherapy Opdivo® (nivolumab) in first-line treatment of advanced HCC.
Other clinical studies of Pexa-Vec include Part 2 of JX594-REN026 (NCT03294083), a Phase Ib trial assessing the candidate in combination with Regeneron Pharmaceuticals’ Libtayo®(cemiplimab-rwlc) in metastatic or unresectable renal cell cancer (RCC); and Phase I combination studies with BMS’ Yervoy® (ipililumab) in solid tumors (NCT02977156) ; in the neoadjuvant setting for solid tumors with University of Leeds; and in combination with AstraZeneca’s Imfinzi® (durvalumab) and tremelimumab in colorectal cancer (NCT03206073).
SillaJen had envisioned Pexa-Vec as its first in a series of gene and viral therapeutics it said it was uniquely positioned to advance through partnerships with Transgene and other companies.
“In particular, our first-in-class oncolytic immunotherapy, Pexa-Vec (JX-594), has shown much potential in several pre-clinical and global clinical trials,” SillaJen CEO Eun-Sang Moon, MD, DDS, states in a message posted on the company’s website. “We are excited about the potential that Pexa-Vec holds for cancer patients and oncologists worldwide.”
Investors reacted to the failure of Pexa-Vec with stock selloffs that sent shares of SillaJen skidding 30% today on the KOSDAQ market, to KRW 31,200 ($25.97) a share. Shares of Transgene, traded on Euronext Paris, fell 12.15% today to €2.03 ($2.25) a share.
Speculation about Pexa-Vec’s clinical performance in PHOCUS arose in July after Shin Hyun-pil, SillaJen’s chief science officer, sold all of his 167,777 common shares of company stock in four transactions, according to a regulatory filing disclosed by the Korea Biomedical Review. The news outlet on July 9 reported the stock sell-off with comment from an unnamed company official that Shin “sold the stocks to pay taxes and personal debt, following the receiving of stock options last year.”




